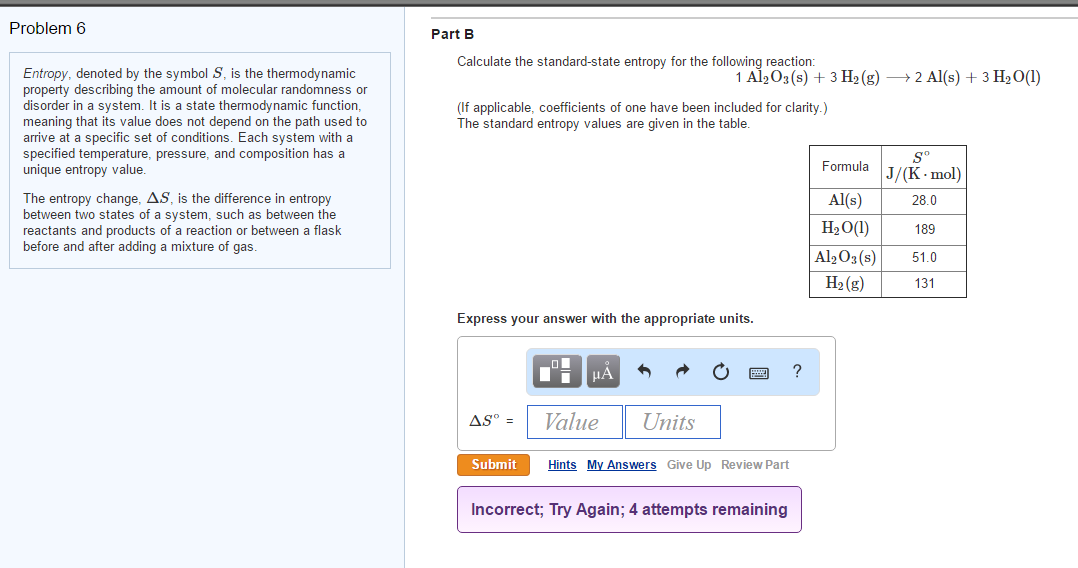
Calculate the entropy change for the following reaction, H2(g) + Cl2(g)⟶ 2HCl(g) at 298 K . Given that, S^ H2 = 131 J K^-1 mol^-1 , S^ Cl2 = 223 J K^-1 mol^-1 and S^ HCl = 187 J K^-1 mol^-1 .

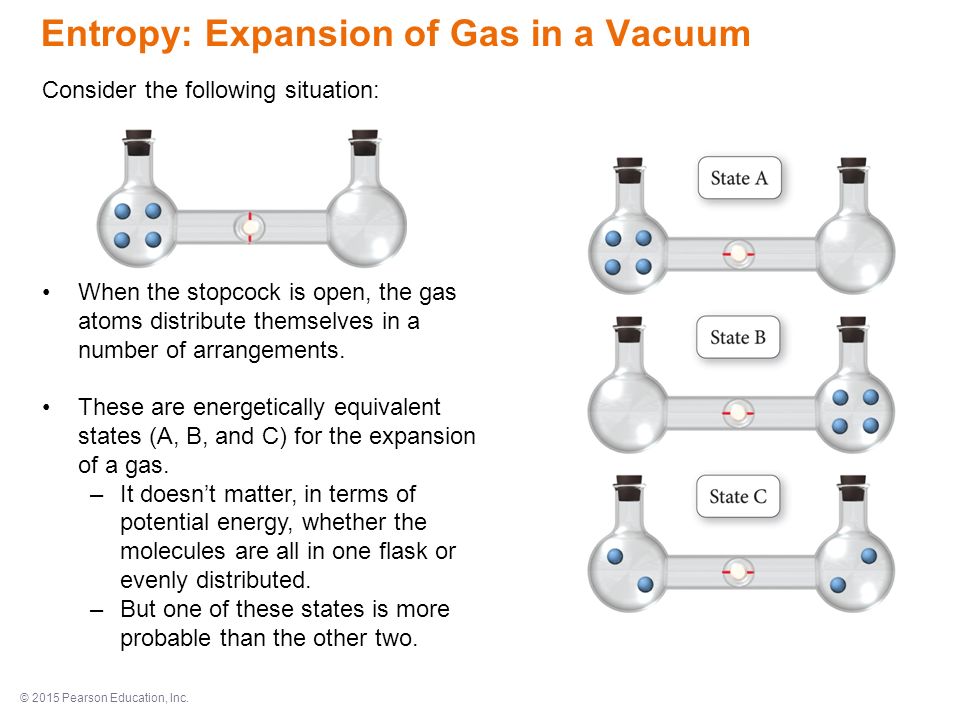
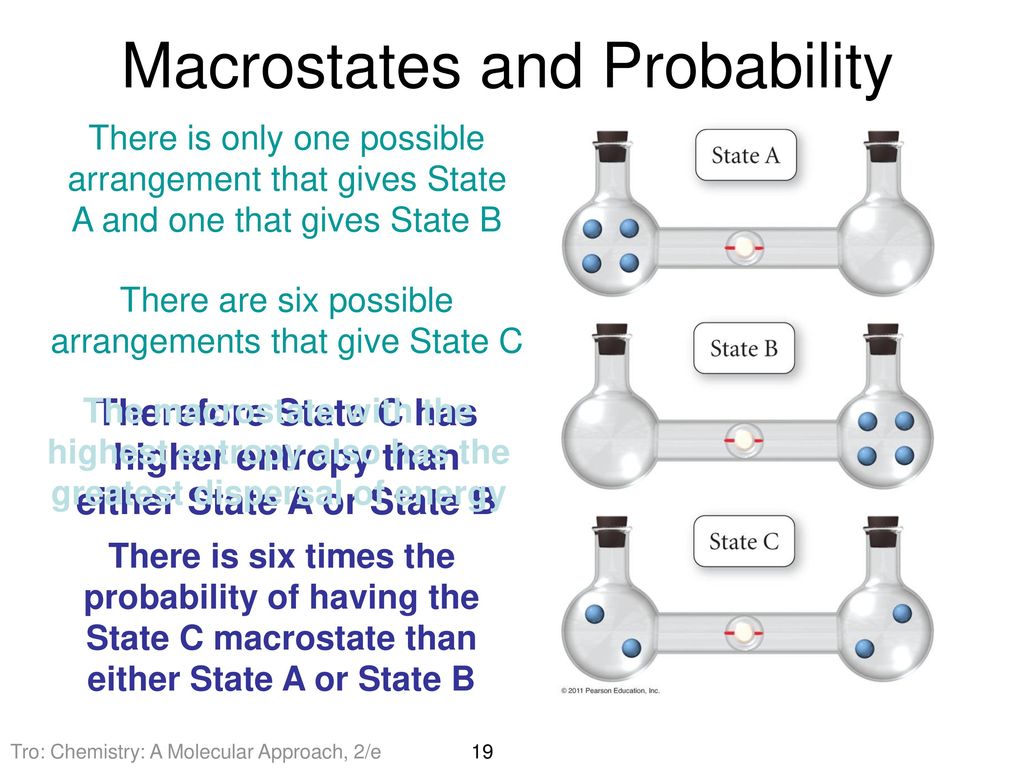
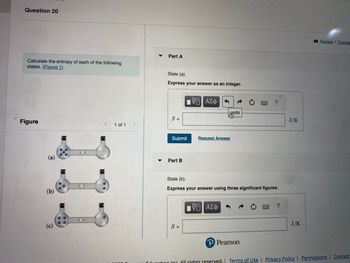
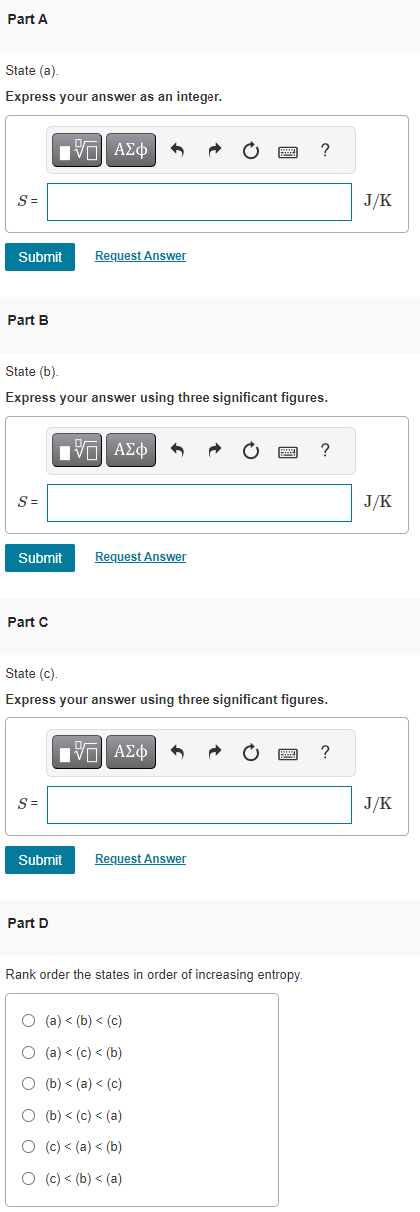
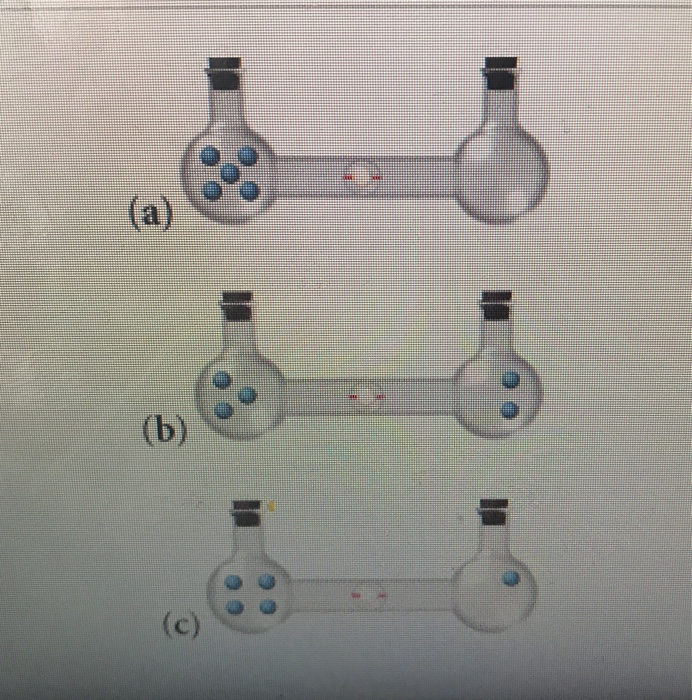
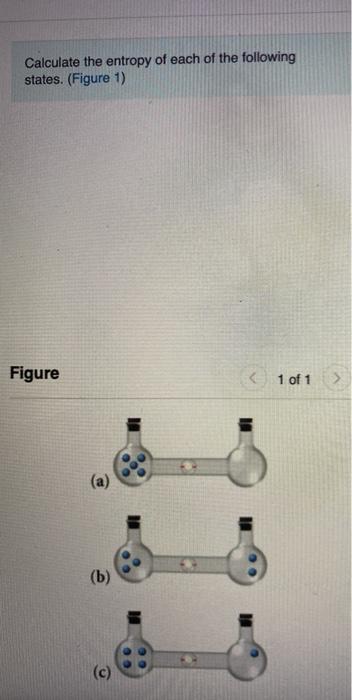
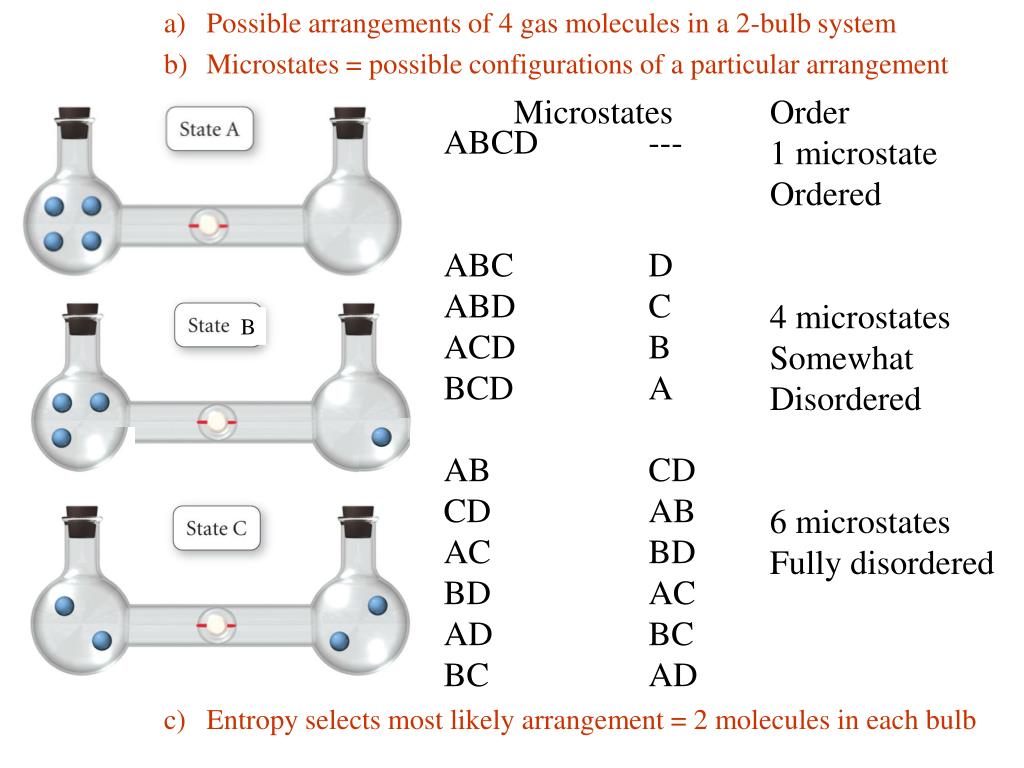
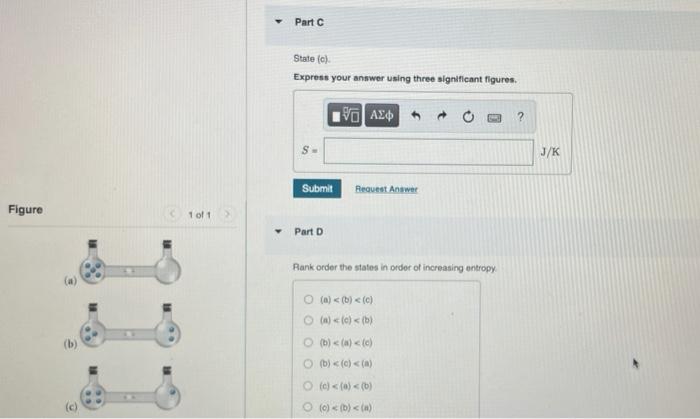
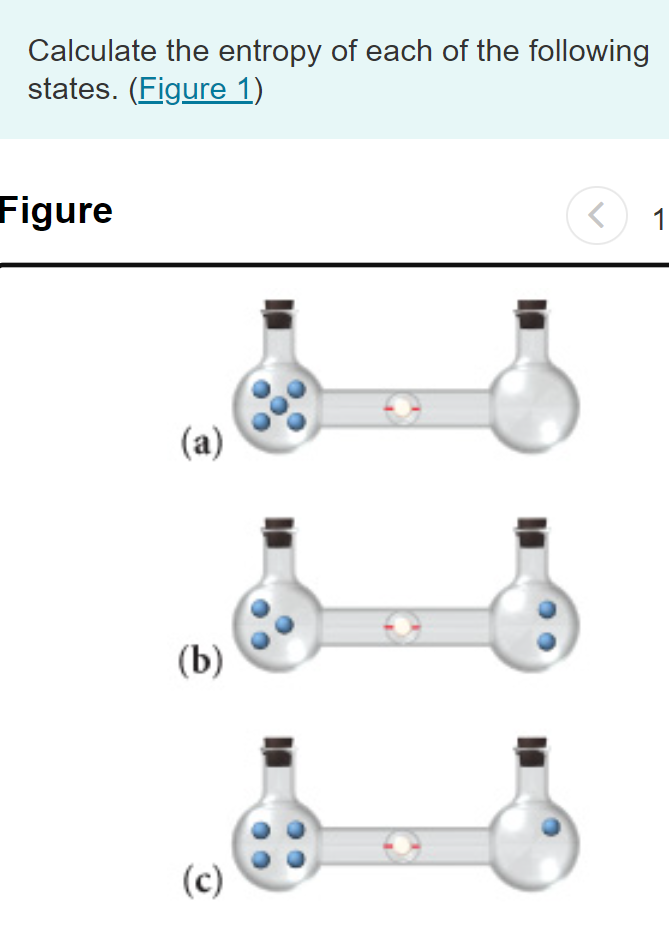
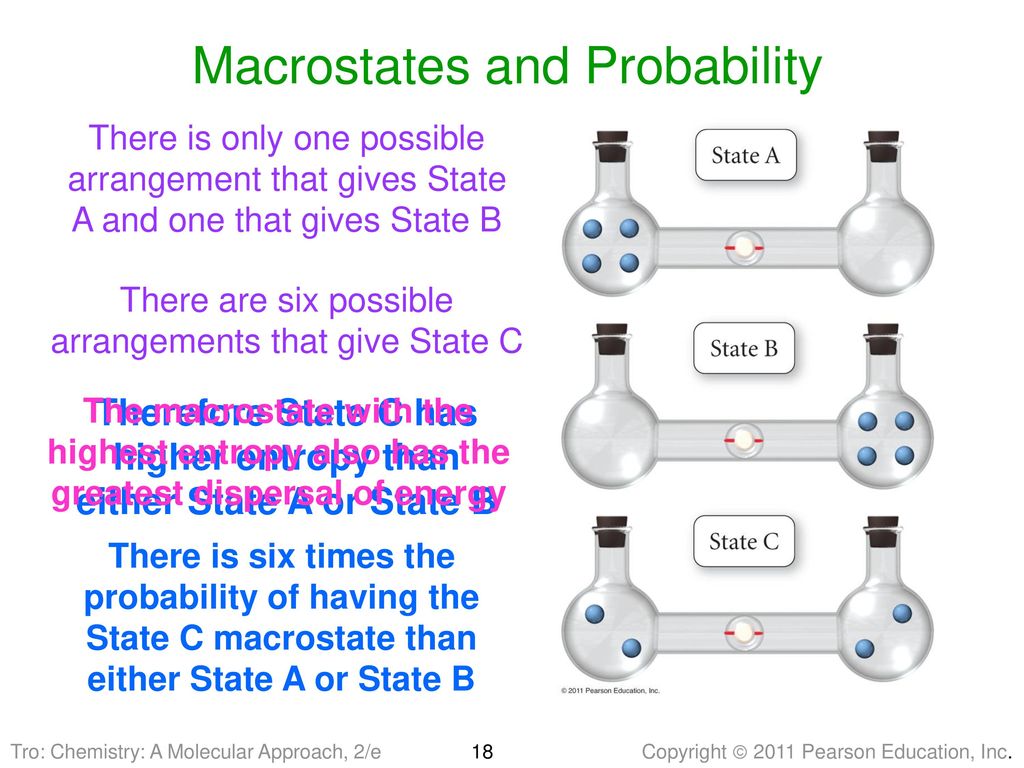
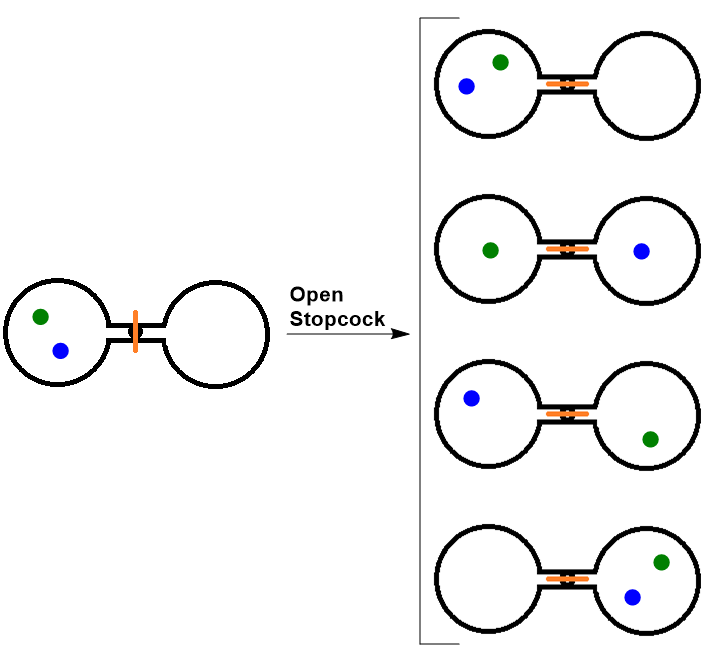
SOLVED:Calculate the entropy of each state and rank the states in order of increasing entropy. a. b. c.
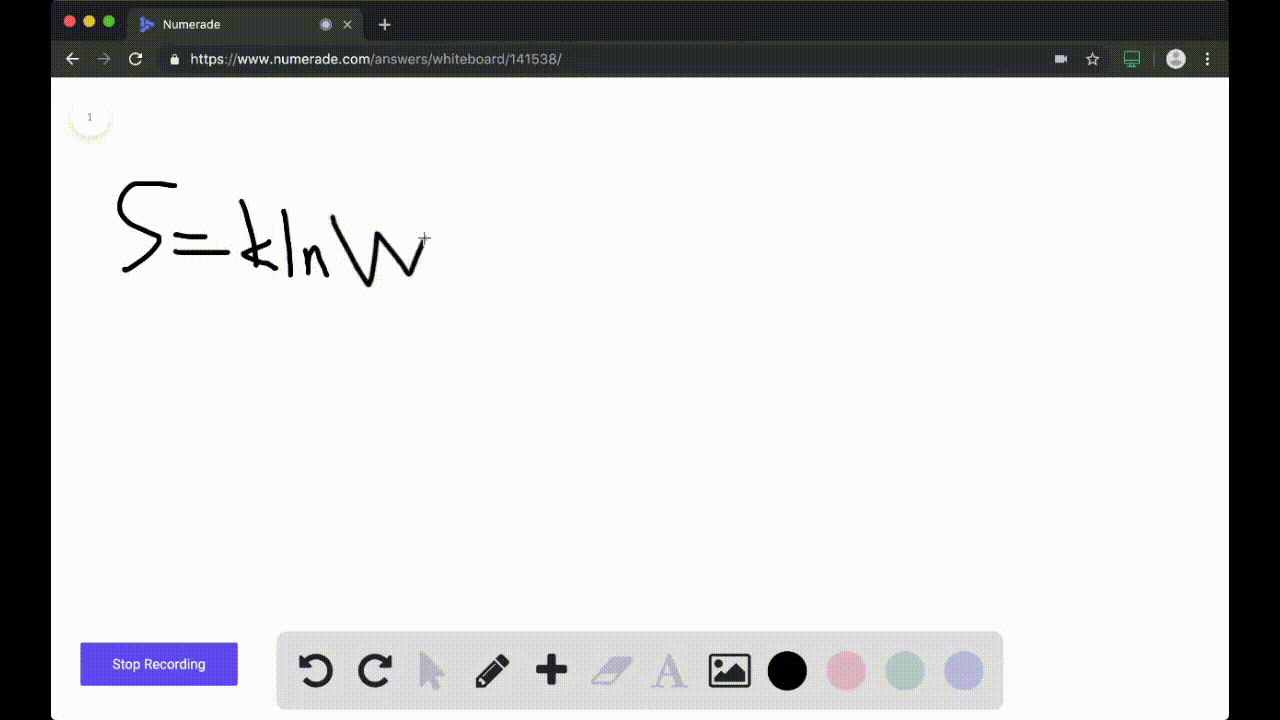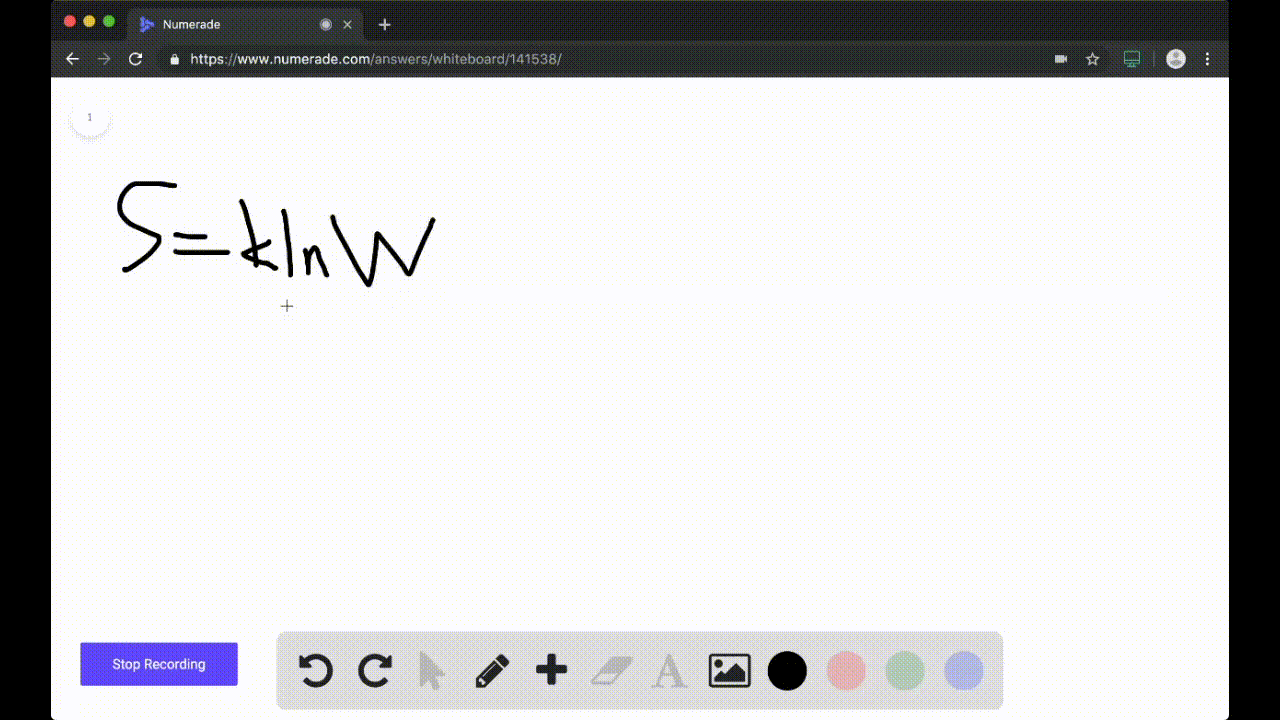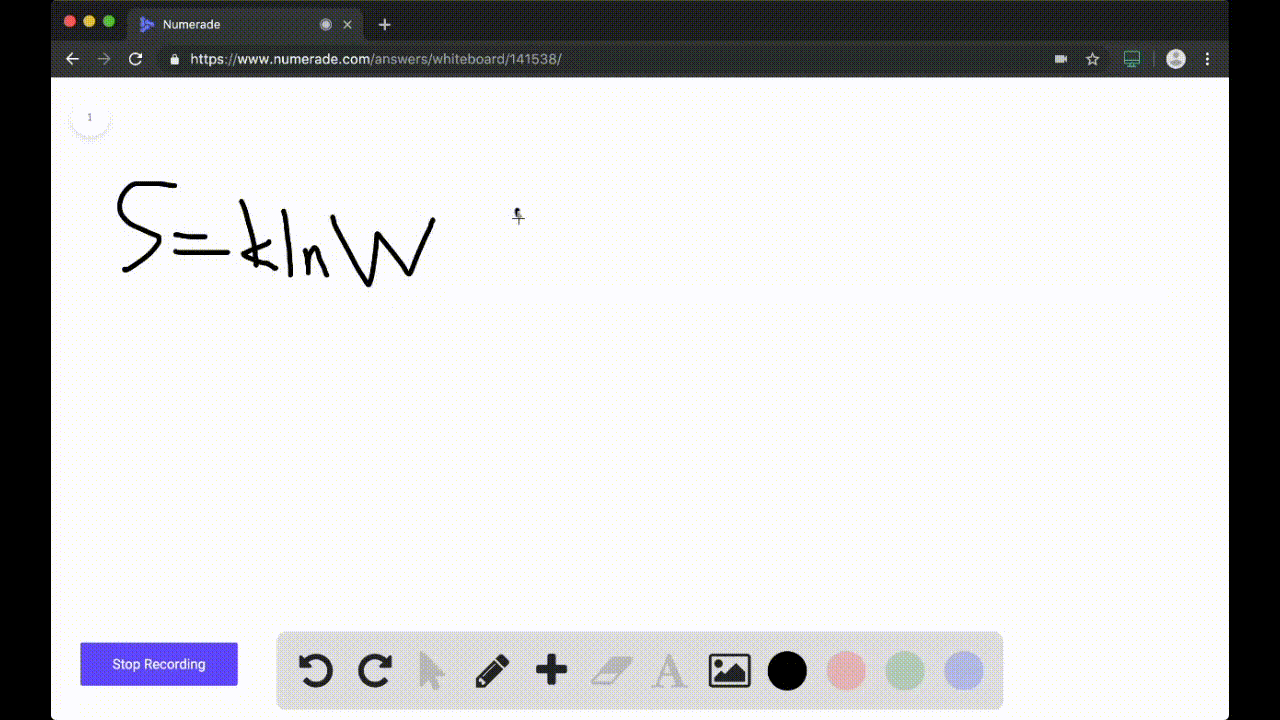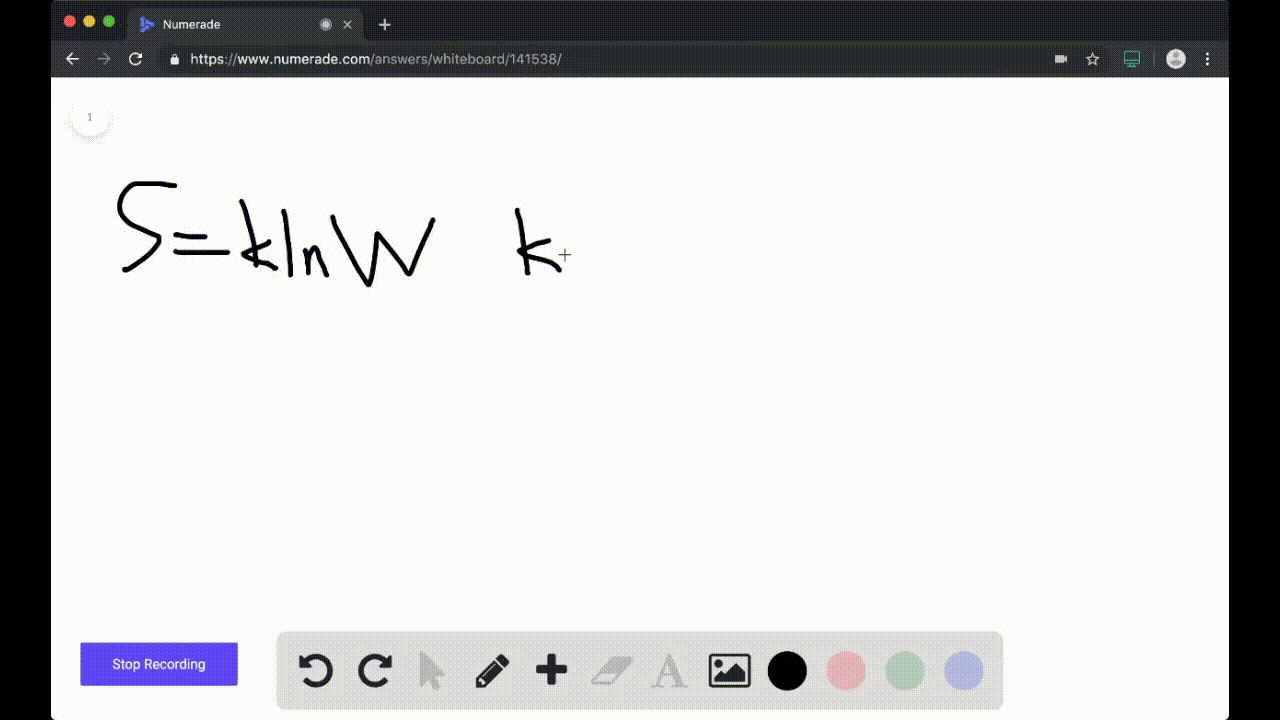
SOLVED:Calculate the entropy of each state and rank the states in order of increasing entropy. a. b. c.

Calculate the entropy change accompanying the following change of state 5 mol of O(2) (27^(@)C, 1 atm) rarr 5 mol of O(2) (117^(@)C, 5 atm) C(P) for O(2) = 5.95 cal deg^(-1) mol^(-1)